Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -octylnitrilo-triacetamide (HONTA) complexes of americium and europium
-octylnitrilo-triacetamide (HONTA) complexes of americium and europiumToigawa, Tomohiro; Peterman, D. R.*; Meeker, D. S.*; Grimes, T. S.*; Zalupski, P. R.*; Mezyk, S. P.*; Cook, A. R.*; Yamashita, Shinichi*; Kumagai, Yuta; Matsumura, Tatsuro; et al.
Physical Chemistry Chemical Physics, 23(2), p.1343 - 1351, 2021/01
Times Cited Count:14 Percentile:81.5(Chemistry, Physical)The candidate An(III)/Ln(III) separation ligand hexa- -octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent (
-octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent ( -dodecane) radical cation of
-dodecane) radical cation of 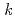 (HONTA + R
(HONTA + R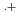 ) = (7.61
) = (7.61  0.82)
0.82)  10
10 M
M s
s obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed
obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed  -dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived (
-dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived ( 100 ns) HONTA radical cation as well as a longer-lived (
100 ns) HONTA radical cation as well as a longer-lived ( s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0501, p.24 - 25, 2020/12
This report summarizes the results obtained in FY2019 at Electron Linac Facility of University of Tokyo. The radiolysis process of a diglycolamide extractant, which is expected to be used in the separation process of minor actinides (MA), in dodecane and octanol solutions was investigated by pulse radiolysis. As a result, it was suggested that by adding alcohol, the decomposition process of the diglycolamide extractant was different from the decomposition processes in the single solvent of dodecane considered that the decomposition occurred via a radical cation species of the extractant.
Yoshida, Yoichi*; Yang, J.*; Kondo, Takafumi*; Seki, Shuhei*; Kozawa, Takahiro*; Tagawa, Seiichi*; Shibata, Hiromi*; Taguchi, Mitsumasa; Kojima, Takuji; Namba, Hideki
JAEA-Review 2005-001, TIARA Annual Report 2004, p.183 - 185, 2006/01
A heavy-ion-pulse radiolysis technology was developed using a single-photon-counting system. In the system, the ion beam was injected a thin scintillator before irradiating the sample. The light emitted from the scintillator by the ion irradiation was used as analyzing source to detect the absorption of primary species in water. Measurement of time-dependent absorption of hydrated electrons in water was achieved using the system, which demonstrates the usefulness of this technique.
Kawanishi, Shuichi; Hagiwara, Miyuki; *; *; *
Radiation Physics and Chemistry, 26(6), p.705 - 713, 1985/00
no abstracts in English
 , He
, He
 , C
, C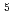
 and Ne
and Ne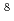
 ions
ionsIwamatsu, Kazuhiro; Yamashita, Shinichi*; Taguchi, Mitsumasa; Kimura, Atsushi; Kurashima, Satoshi; Katsumura, Yosuke
no journal, ,
Heavy ion beams, one of the high linear energy transfer (LET) radiations induce specific irradiation effects which are different from those of low LET radiations. The effects are attributed to heterogeneous distribution of reactive species along their trajectories, so called "track structure". Water was selected as target in this study because more data exist for radiolysis than any other substances. Hydroxyl radical (OH), one of the most important water decomposition species, was focused on by using bromide ion as a probing reagent, and their reactions were observed by the ion beam pulse radiolysis system. The formation and decay of Br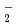 were observed at 375 nm (
were observed at 375 nm ( [Br
[Br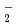 ] = 9000 M
] = 9000 M
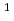 cm
cm
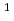 ). The formation chemical yield of it after pulse irradiation decreased with increasing atomic numbers of the incident ions. Radical recombination reaction occurs easier by higher LET ions because of denser radical formations. Therefore, the decrease in the yields of OH scavenged by Br
). The formation chemical yield of it after pulse irradiation decreased with increasing atomic numbers of the incident ions. Radical recombination reaction occurs easier by higher LET ions because of denser radical formations. Therefore, the decrease in the yields of OH scavenged by Br induces the decrease in the chemical yields of Br
induces the decrease in the chemical yields of Br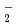 . The decay rates of the chemical yields increased with the atomic mass of the ions. The concentrations depend on the dose rate and chemical yield, and the dose rates proportional to LET value. The concentrations after pulse increased with increase in the atomic mass of the ions and resulted in faster decay in heavier ions.
. The decay rates of the chemical yields increased with the atomic mass of the ions. The concentrations depend on the dose rate and chemical yield, and the dose rates proportional to LET value. The concentrations after pulse increased with increase in the atomic mass of the ions and resulted in faster decay in heavier ions.
Toigawa, Tomohiro; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Matsumura, Tatsuro
no journal, ,
The radiolytic degradation of amidic extractants excepted to be used in the minor actinide sepation were observed by using the pulse radiolysis system in The University of Tokyo. It was found that a intermediate specie for degradation was generated not only by direct ionization of the extractant but also derived from the diluent ionization.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Matsumura, Tatsuro
no journal, ,
no abstracts in English